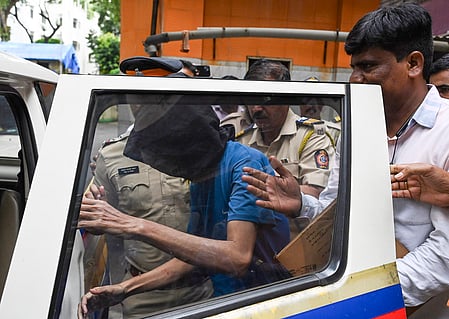Cough syrup deaths: Kerala, MP suspend sale of Coldrif syrup, TN imposes ban; CDSCO launches inspection
11 children in Chhindwara district of Madhya Pradesh reportedly died due to consuming Coldrif Cough syrup. A campaign has since been underway to seize Coldrif syrup across the state.

AFTER reports of child deaths due to the consumption of contaminated Coldrif cough syrup, Madhya Pradesh and Kerala suspended the sale of the cough syrup, while Tamil Nadu imposed a total ban on it.
Meanwhile, the health ministry said that the central drug regulator, CDSCO, has initiated risk-based inspection at the manufacturing units across six states.
11 children in Chhindwara district of Madhya Pradesh reportedly died due to consuming Coldrif Cough syrup. A campaign has since been underway to seize Coldrif syrup across the state.
“The families of the 11 children who died in Chhindwara will receive financial assistance of ₹4 lakh each. The state government will also bear the entire cost of treatment for the children,” said Madhya Pradesh CMO.
CDSCO launches inspection
Central Drugs Standard Control Organisation (CDSCO) began risk-based inspection at the manufacturing units of 19 drugs, including cough syrups and antibiotics, across six states on Friday, October 3
These inspections aim to identify gaps that may have led to drug quality failures and suggest process improvements to avoid such incidents in the future.
Additionally, the health ministry said that a multidisciplinary team comprising experts from the National Institute of Virology, the Indian Council of Medical Research, National Environmental Engineering Research Institute, CDSCO and AIIMS-Nagpur, among others, are still analysing the various samples and factors to assess the cause of deaths in and around Chhindwara in Madhya Pradesh.
Six drug samples tested by CDSCO and three by the Madhya Pradesh Food and Drugs Administration (MPFDA) were found to be free of Diethylene Glycol (DEG) and Ethylene Glycol (EG) contaminants that are known to cause serious kidney injury.