Cancer fear, health risk — US recalls blood pressure medicine
The FDA said drugmakers have recalled a staggering amount of blood pressure medication prazosin hydrochloride. Here's all you need to know about it.
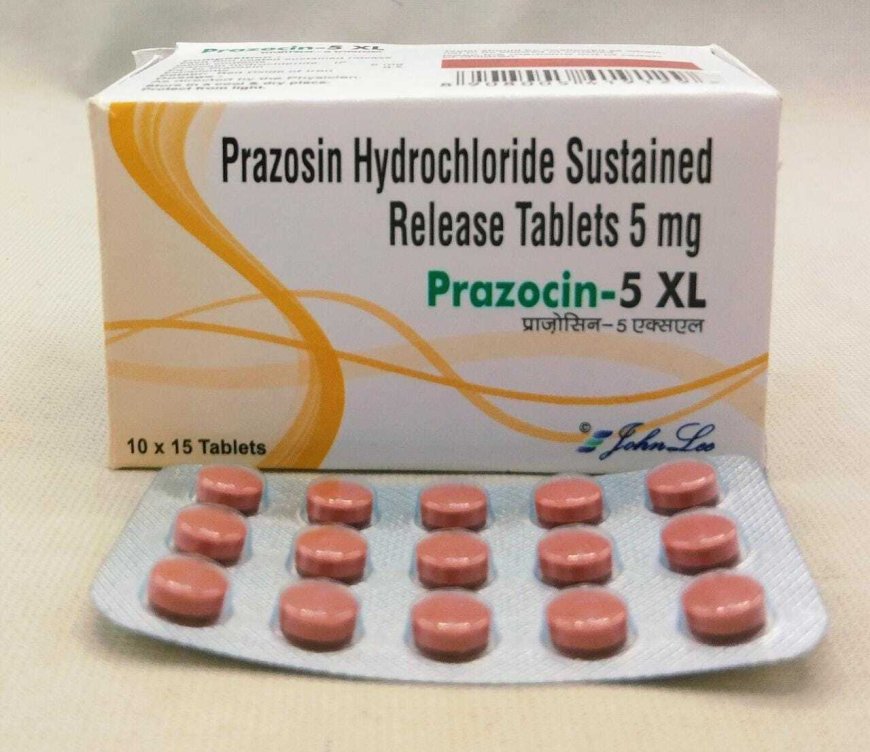
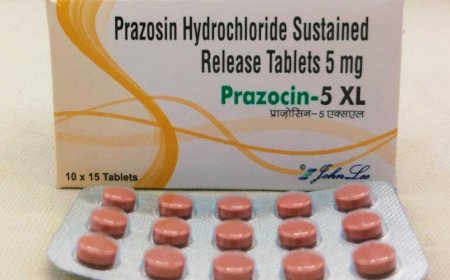
THE US Food and Drug Administration (FDA) on Friday said drugmakers have recalled a staggering amount of blood pressure medicine prazosin hydrochloride, an alpha-blocker that works by relaxing blood vessels, over concerns that it might contain a cancer-causing impurity.
Earlier this month, the FDA said that New Jersey-based Teva Pharmaceuticals USA and drugs distributor Amerisource Health Services issued voluntary nationwide recalls of over 580,000 bottles of various strengths of prazosine capsules, reported AP.
What are prazosin hydrochloride capsules?
Prazosin hydrochloride is a popular blood pressure medicine and belongs to the drug class of Alpha-1 adrenergic blocker. It helps to relax blood vessels.
Doctors prescribe prazosin to help lower blood pressure. It is also sometimes prescribed for nightmares and other sleep disturbances caused by post-traumatic stress disorder.
What happened in US?
US-based Teva Pharmaceuticals voluntarily recalled over 580,000 bottles of various strengths of prazosine capsules.
A 'voluntary recall' means the recall was not ordered by the FDA; instead, the prazosine capsules were voluntarily pulled from the market and stores by the companies and the drug distributors.
Teva issued the voluntary recall on 7 October.
Why the blood pressure medication is recalled
The blood pressure medicines were recalled over concerns they may contain a cancer-causing compound.
According to the FDA report, the recalled drug contained nitrosamine impurities - identified as “N-nitroso Prazosin impurity C”.
Nitrosamines are classified as possible human carcinogens, and long-term exposure to them could increase the risk of cancer in patients consuming the drug.
Also Read | Why are these 2 popular candies being recalled in New York ahead of Halloween?
The FDA also mentioned in enforcement orders posted online that it has given the affected lots of the drug a Class II risk classification. Class II classified drugs mean that the drugs, if consumed, could cause “temporary or medically reversible health issues”, though serious harm is unlikely.
Which are the affected lots?
As per the FDA’s recall notice, the affected blood pressure medicine products of prazosin hydrochloride are as follows:
181,659 bottles of 1 mg capsules
291,512 bottles of 2 mg capsules
107,673 bottles of 5 mg capsules
Each bottle can contain between 100 and 1,000 capsules, depending on the packaging.
What to do if medicine is recalled?
Teva Pharmaceuticals advised those who take the medication to contact their pharmacy to determine what to do with the remaining quantities. Teva also said it sent recall letters to its customers with instructions for returning the recalled product.
"In addition, consumers with questions or concerns should also contact the health care provider who prescribed the medication," the drug distributor said in its statement.